Are you wondering what makes one crystal red and another one blue? How do crystals get their colors?
Crystals reflect the wavelengths in light to get color. For example, the structure and chemical elements in a red crystal reflect red light wavelengths back to your eyes, so the crystal looks red to you.
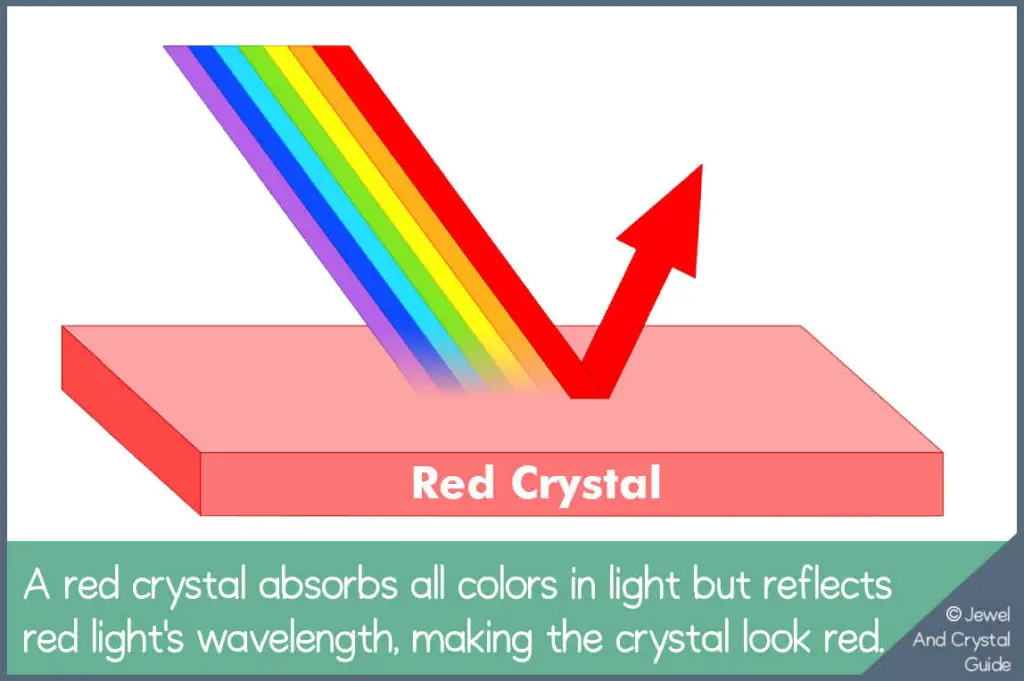
This is the simplest way to explain crystal colors. To truly understand how crystals get their colors, we need to think about what makes up light and what happens inside a crystal when light hits it. Once we know this, things get interesting because crystals can also change color or hue. Keep reading to find out more…
In this blog post, you’ll discover:
- How crystals get their colors
- What changes a crystal’s color and why
How Crystals Get Their Colors
Light gives us all the colors
White light may look white to us, but it’s actually made up of all the colors of the rainbow: violet, indigo, blue, green, yellow, orange, and red. Each color is on a different wavelength, but when they’re all together they look white.
When white light hits an object, the atoms, molecules, or ions in that object interact with the incoming light.
- Atoms are the building blocks of matter. Their central nucleus houses protons and neutrons, and it’s surrounded by electrons in orbit.
- A molecule is the smallest unit of a chemical compound that consists of two or more atoms chemically bonded together.
- Ions are electrically charged particles that form when atoms or molecules gain or lose electrons, resulting in either a positive or negative charge.
Some of these atoms, molecules, or ions absorb certain wavelengths of light (i.e. certain colors). When they absorb colors in white light, those colors are “taken out” of the light, leaving the rest of the colors to be reflected.
So the color we see when we look at an object is the color that object reflects or transmits.
For example, a banana looks yellow because it absorbs most of the colors in white light, except yellow. The banana reflects the yellow wavelengths back to your eyes, so you see a banana that is yellow.
Crystals get their colors in much the same way. But there are two things that decide what wavelengths a crystal absorbs and reflects:
- The atoms, ions, and molecules that make up the crystal, and
- The way in which atoms, ions, and molecules are arranged inside the crystal.

Let me explain each of these in a way that’s easy to understand.
How atoms, ions, and molecules give crystals color
As we saw above, different atoms, molecules, or ions absorb and reflect different wavelengths of light.
Imagine you’re baking cupcakes for a party and you add green food coloring to the mix. Now all of the cupcakes look green because the food coloring in them reflects green wavelengths in light. The food coloring determines the color of the cake.
Well, atoms, ions, and molecules work in much the same way as the food coloring.
The types of atoms, molecules, or ions in a crystal determine what colors the crystal can be. If the atoms, molecules, or ions reflect blue light, then the crystal will appear blue. If the atoms, molecules, or ions reflect red light, then the crystal will appear red. And so on.
How the crystal’s structure determines its color
Atoms and ions are neatly arranged in a crystal’s structure. They’re held together by invisible forces, and they repeat in a pattern.
Crystals with a well-ordered and regular atomic structure are often clear or transparent. This is because the regular arrangement of atoms or ions lets light through without much scattering or absorption.
So light that hits a crystal with a highly ordered structure moves through it without much disruption, resulting in transparency and a crystal that is quite see-through.
But even a well-structured crystal can have color and color variations. Let’s find out about the 7 things that affect a crystal’s color.
7 Things That Affect A Crystal’s Color
Did you know that crystals can change color, get color spots, or change shades when they’re exposed to certain things, like chemicals, impurities, different environments, heat, or pressure?
This is one of the reasons why you need to be careful when charging crystals in sunlight or putting crystals in water.
Here are 7 things that can change the color of a crystal:
Structural defects as a crystal forms
Crystals have a highly ordered, repeating internal structure. If you look at a crystal through a special scientific microscope, you would see the crystal’s atoms and ions in a repeating pattern.
But when some crystals form in nature, they get defects in their structure. Sometimes the pattern gets broken, some atoms go missing, extra atoms get added to the structure, the atoms don’t line up as they should, or impurities get into the structure (see more about impurities below).
These structural irregularities often change the way the crystal absorbs and reflects light, which then changes the color we see when we look at the crystal.
For example, structural defects can happen in diamonds when carbon atoms are missing from their regular structural positions. Sometimes these missing atoms get replaced by impurities. Diamonds with structural defects can turn many different colors, such as blue, green, pink, and brown, depending on the defect and impurities present.
Impurities that get into the crystal structure
An impurity is anything that shouldn’t be in a crystal. Remember that this is specific to the crystal because what should and shouldn’t be in one crystal is not necessarily what should and shouldn’t be in another crystal.
So impurities are foreign elements or compounds that find their way into a crystal’s structure, often in very small amounts.
Impurities change a crystal’s color because they can change the way the crystal absorbs and reflects light. The type of impurity and its concentration determine what color the crystal becomes and how intense the color is.
For example, the metal impurity chromium is what gives rubies their bright red color.
These color changes can sometimes change the type of crystal it is too. For example, iron impurities in quartz change its color from clear to purple, turning the quartz into an amethyst!
Irradiation changes the crystal’s structure
Irradiation happens when crystals are exposed to various forms of ionizing radiation, such as gamma rays, X-rays, or even high-energy particles from radioactive materials. Natural ionizing radiation comes from sources like cosmic rays and radioactive elements in the Earth’s crust.
This radiation carries enough energy to interact with the atoms, molecules, or ions in the crystal’s structure:
As ionizing radiation passes through a crystal, it can dislodge or ionize (change the charge of) atoms or electrons inside the crystal. This can create color centers, gaps where atoms or ions should be, and other structural defects.
Smoky quartz is an example of this as the crystal gets its smoky brown or gray color from natural radiation exposure. Over time, the radiation causes defects in the quartz structure, which trap electrons and give it the smoky coloration.
Controlled heat treatment to change a crystal’s color
Heat treatment is mainly done by people to improve the color and clarity of some minerals, especially if they can sell them for more money after treatment.
Crystals get exposed to controlled high temperatures to remove or reduce impurities and change the distribution of color-causing elements in the crystals.
This changes the crystals’ color or improves their clarity. Clarity is how much light passes through a crystal or gemstone, and a general rule of thumb is that the better the clarity, the more valuable the piece is.
Inclusions that affect the color and hue of a crystal
As a crystal grows, it can trap small foreign crystals, gas bubbles, or even liquid droplets within its structure – and you can sometimes even see these inside the crystal. These are called inclusions.
So inclusions are any solids or liquids trapped inside a crystal.
They can change a crystal’s appearance, including its color and shade, based on the type of inclusion, the crystal’s structure, the light source, and the viewing angle.
Pressure-induced color changes
High-pressure conditions in the Earth can change a crystal’s structure, which affects its color or shade.
This usually happens because pressure compresses the crystal, changing the distances between atoms or ions in the structure. This then changes the way the crystal absorbs and reflects light, which alters its color and hue.
So, a crystal that is one color under normal conditions can become a different color under pressure. For example, diamonds often turn pink under extreme pressure.
But pressure can also make a crystal’s color darker or lighter, where the crystal keeps its original color but the color changes in intensity.
Sometimes the color change is reversible, other times it isn’t.
Environment
The environmental conditions under which crystals are stored or displayed can affect their colors and intensity.
Crystals that are exposed to things like wind, sunlight, heat, or moisture over a long period of time can get a damaged surface, fade, or even change color.
For example amethyst, a purple variety of quartz, fades to a pale yellow or colorless state when exposed to prolonged sunlight or high heat.
Want to remember this? Save this post on How Crystals Get Colors to your favorite Pinterest Board below!










